Pfizer Inc Patent Portfolio Statistics
Profile Summary
This article summarizes the perfomance of the assignee in the recent years. The overall statistics for this portfolio help to analyze the areas where the assignee is performing well. The filing trend, perfomance across the tech centers and the perfomance of the recent applications has been mentioned below. All the stats are calculated based on the perfomance in USPTO.
How does the overall patent portfolio of Pfizer Inc. look like?
| Assignee | Art Units | |
| Total Applications: | 3,142 | 1,752,003 |
| Granted Patents: | 1,218 | 910,450 |
| Grant Index | 66.96% ↑ | 66.03% |
| Abandoned/Rejected Applications: | 601 (33.04%) | 468,451 (33.97%) |
| In-Process Applications: | 1,321 | 373,102 |
| Average Grant Time: | 3.0 Years ↑ | 2.78 Years |
| Average Office Actions: | 1.87 ↑ | 1.69 |
Which Technology Area Pfizer Inc. is filing most patents in? (Last 10 years)
| Art Unit | Definition | Total Applications |
| 1624 | Organic Chemistry | 218 |
| 1625 | Organic Chemistry | 188 |
| 1626 | Organic Chemistry | 140 |
| 1614 | – | 90 |
| 1648 | Immunology, Receptor/Ligands, Cytokines Recombinant Hormones, and Molecular Biology | 90 |
How many patents are Pfizer Inc. filing every year?
| Year | Total Applications | Predicted |
| 2022 | 0* | 57 |
| 2021 | 7* | 44 |
| 2020 | 25 | 48 |
| 2019 | 34 | 34 |
| 2018 | 27 | – |
| 2017 | 31 | – |
| 2016 | 41 | – |
| 2015 | 32 | – |
| 2014 | 34 | – |
| 2013 | 41 | – |
*The drop in the number of applications filed in last two years compared to previous years is because applications can take up to 18 months to get published
Recently filed patent applications of Pfizer Inc. in USPTO?
Application number: 17/544,303
Abstract:
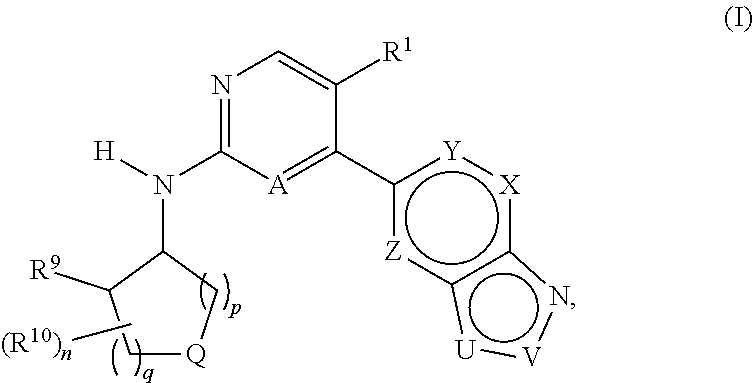
or a pharmaceutically acceptable salt thereof, in which R-groups R1 to R23, A, Q, U, V, W, X, Y, Z, n, p and q are as defined herein, to pharmaceutical compositions comprising such compounds and salts, and to methods of using such compounds, salts and compositions for the treatment of abnormal cell growth, including cancer, in a subject.
Publication date: 2022-03-24
Applicant: Pfizer Inc.
Inventors: Cho-Schultz Sujin
Publication number: US20220089613A1
Application number: 17/539,948
Abstract:
Publication date: 2022-03-24
Applicant: Pfizer Inc.
Inventors: Nukui Seiji
Publication number: US20220207454A1
Application number: 17/607,341
Abstract:
Publication date: –
Applicant: Pfizer Inc.
Inventors: David Mcgettigan Scott
How are Pfizer Inc.’s applications performing in USPTO?
| Application Number | Title | Status | Art Unit | Examiner |
| 17/544,303 | Cyclin Dependent Kinase Inhibitors | Docketed New Case – Ready for Examination | OPAP | Central, Docket |
| 17/539,948 | Novel 3-Amino-Pyrrolo[3,4-C]Pyrazole-5(1H, 4H,6H) Carbaldehyde Derivatives | Docketed New Case – Ready for Examination | OPAP | Central, Docket |
| 17/607,341 | Real-Time Tracking And Management Of Standard Workflows | Docketed New Case – Ready for Examination | OPAP | Central, Docket |

